摘要:
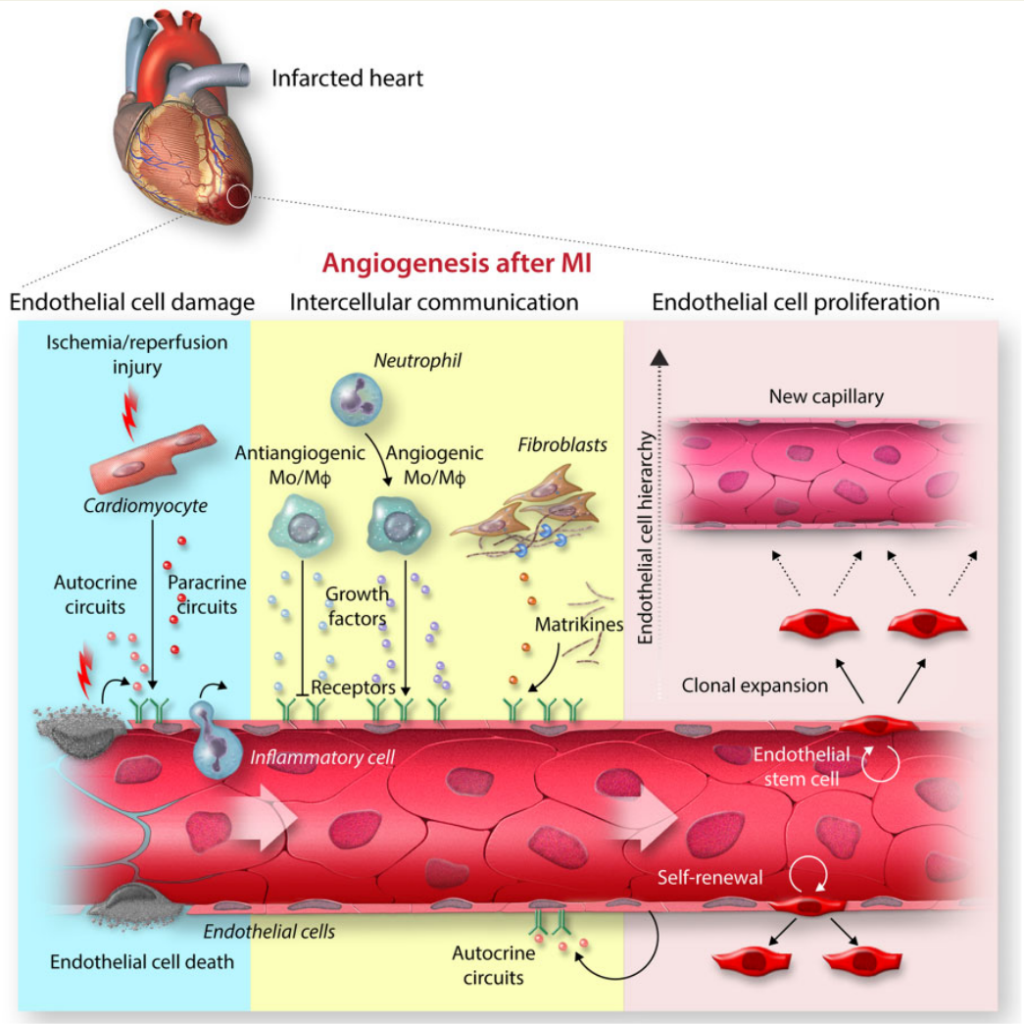
Acute myocardial infarction (MI) inflicts massive injury to the coronary microcirculation leading to vascular disintegration and capillary rarefication in the infarct region.
急性心肌梗塞(MI)对冠状动脉微循环造成巨大损伤,导致梗塞区域的血管崩解和毛细血管稀疏。
vascular disintegration 血管解体 血管崩解 capillary 毛细血管 rarefication 稀疏
Tissue repair after MI involves a robust angiogenic response that commences in the infarct border zone and extends into the necrotic infarct core.
angiogenic 血管生成 commences in the 开始于
心肌梗死后的组织修复包括强有力的血管生成反应,该反应始于梗死边缘区并延伸至坏死的梗死核心区。
Technological advances in several areas have provided novel mechanistic understanding of postinfarction angiogenesis and how it may be targeted to improve heart function after MI.
几个领域的技术进步提供了对心肌梗死后血管新生的新的机制理解,以及它如何被用于改善心肌梗死后的心功能。
Cell lineage tracing studies indicate that new capillary structures arise by sprouting angiogenesis from pre-existing endothelial cells (ECs) in the infarct border zone with no meaningful contribution from non-EC sources.
细胞谱系追踪研究表明,新的毛细血管结构是由梗塞边缘区中预先存在的内皮细胞(EC)萌发的血管生成产生的,非EC来源没有有意义的贡献。
Cell lineage tracing 细胞谱系追踪
Single-cell RNA sequencing shows that ECs in infarcted hearts may be grouped into clusters with distinct gene expression signatures, likely reflecting functionally distinct cell populations.
单细胞RNA测序显示,梗死心脏中的内皮细胞可以分成具有不同基因表达特征的簇,这可能反映了功能不同的细胞群。
EC-specific multicolour lineage tracing reveals that EC subsets clonally expand after MI. Expanding EC clones may arise from tissue-resident ECs with stem cell characteristics that have been identified in multiple organs including the heart.
内皮细胞特异性多色谱系追踪显示内皮细胞亚群在心梗后增殖。内皮细胞增殖可能来源于具有干细胞特征的组织驻留内皮细胞,这种细胞已经在多种器官中表征,包括心脏。
内皮细胞特异性多色谱系追踪显示心肌梗死后内皮细胞亚群克隆性扩展。扩增的内皮细胞克隆可能来自具有干细胞特征的组织常驻内皮细胞,这些干细胞特征已在包括心脏在内的多个器官中得到鉴定。
Tissue repair after MI involves interactions among multiple cell types which occur, to a large extent, through secreted proteins and their cognate receptors.
心肌梗死后的组织修复涉及多种细胞类型之间的相互作用,这在很大程度上通过分泌蛋白及其同源受体发生。
secreted proteins 分泌蛋白 cognate receptors 同源受体
While we are only beginning to understand the full complexity of this intercellular communication, macrophage and fibroblast populations have emerged as major drivers of the angiogenic response after MI.
虽然我们才刚刚开始理解这种细胞间通讯的全部复杂性,但巨噬细胞和成纤维细胞群已经成为心肌梗死后血管生成反应的主要驱动因素。
Animal data support the view that the endogenous angiogenic response after MI can be boosted to reduce scarring and adverse left ventricular remodelling.
动物数据支持这样的观点,即心肌梗死后内源性血管生成反应可被加强,以减少疤痕和不利的左心室重塑。
scarring 疤痕 left ventricular remodelling 左室重构
The improved mechanistic understanding of infarct angiogenesis therefore creates multiple therapeutic opportunities.
因此,对梗死血管生成的机制理解的提高创造了多种治疗机会。therapeutic治疗的
During preclinical development, all proangiogenic strategies should be tested in animal models that replicate both cardiovascular risk factor(s) and the pharmacotherapy typically prescribed to patients with acute MI.
在临床前开发阶段,所有促血管生成策略都应在复制心血管危险因素和急性心肌梗死患者常用药物疗法的动物模型中进行测试。
Considering that the majority of patients nowadays do well after MI, clinical translation will require careful selection of patients in need of proangiogenic therapies.
考虑到如今大多数患者在心梗后恢复良好,临床转化将需要仔细选择需要促血管生成治疗的患者。